Tailored synthesis of RuO2-V2O5 nanocomposites by controlled spray pyrolysis: Unlocking high-efficiency energy storage electrodes
- Journal
- Journal of Alloys and Compounds
- Vol. (No.), pp.
- 1050, 185542(Jan 2026)
- Year
- 2025
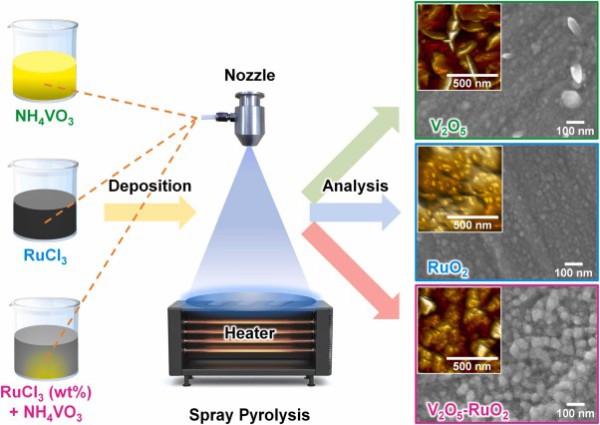
The tailored synthesis of vanadium pentoxide (V2O5)-based electrodes holds immense promise for advancing high-performance electrochemical energy storages. However, the practical use of V2O5 is hindered by its inherently low electrical conductivity and structural degradation during prolonged cycling. To address these essential challenges, ruthenium dioxide (RuO2) is strategically integrated as a secondary oxide to form hybrid RuO2-V2O5 nanocomposites through a controlled spray pyrolysis. In this process, ruthenium precursors with various concentrations (0.5–10 wt%) are incorporated into a vanadium precursor solution, followed by the deposition onto the preheated stainless-steel substrates, to tailor the morphology of the resulting thin-film nanocomposite (thickness <10 μm). The optimized RuO2-V2O5 nanostructures exhibit excellent hydrophilicity and feature a unique architecture of two-dimensional RuO2 nanosheets uniformly dispersed over the V2O5 matrix. This configuration establishes RuO2-V2O5-interconnected conducting pathways that significantly enhance charge transport and efficient ion diffusion. Remarkably, the 6 wt% RuO2-V2O5 electrode achieves a high specific capacitance of 1223.48 F g−1 (734.09 C g−1) in a 6 M KOH at 0.1 A g−1. Furthermore, an asymmetric device fabricated with the optimized RuO2-V2O5 electrode delivers an impressive energy density of 14.40 Wh kg−1 and a power density of 35.71 kW kg−1, while maintaining ∼98 % efficiency and 85.34 % capacitance retaining over 10,000 cycles. These findings highlight a promising route for fabricating stable and scalable nanocomposite electrodes for practical energy storage systems.